When Rubidium Metal Is Exposed to Air
Rubidium forms salts with halogens producing rubidium fluoride rubidium chloride rubidium bromide and rubidium iodide. The heat produced by this reaction may ignite the hydrogen or the metal itself resulting in a fire or an explosion.

Did You Know Hummingbird Is The Only Bird That Can Fly Backwards The Hummingbird Has A Unique Muscle And Wing Structure Th Did You Know D I D Wing Structure
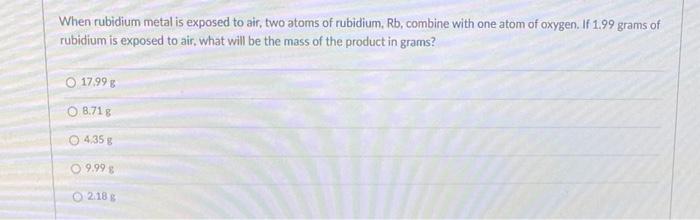
When rubidium metal is exposed to air two atoms of rubidium Rb combine with one atom of oxygen.

. The most common and widely. Ordinary rubidium is sufficiently radioactive to expose a photographic film in about 30 to 60 days. When rubidium metal is exposed to air two atoms of rubidium Rb combine with one atom of oxygen.
Rubidium has also been reported to ignite spontaneously in air. When rubidium metal is exposed to air two atoms of rubidium Rb combine with one atom of oxygen. If 175 grams of rubidium is exposed to air what.
In its elemental form rubidium has a gray white appearance. When rubidium metal is exposed to air one atom of rubidium Rb combines with two atoms of oxygen. Asked Jun 19 2017 in Chemistry by Gabriela.
When rubidium metal is exposed to air two atoms of rubidium Rb combine with one atom of oxygen. Rubidium is highly reactive with properties similar to other Group 1 alkali metals eg rapid oxidation in air. If 175 grams of rubidium is exposed to air what will be the mass of the product in grams.
When rubidium metal is exposed to air two atoms of rubidium Rb combine with one atom of oxygen. The ionization energy of rubidium is very low 406 kJmol. Rubidium in excess oxygen gives the superoxide RbO 2.
It forms alloys with iron gold sodium and potassium and forms amalgams with mercury. It is oxidised forming principally Rb2O. Rubidium is an element with atomic symbol Rb atomic number 37 and atomic weight 85468.
Rb 2 O Rb 2 O 2 Rb 2 O 3 Rb 2 O 4. B 217 g. When rubidium metal is exposed to air two atoms of rubidium Rb combine with one atom of oxygen.
Please show conversions thanks. A 0328 g B 241 g C 208 g D 0655 g E 137 g. The heavier alkali metals rubidium and cesium will spontaneously ignite upon exposure to air at room temperature.
Naturally occurring rubidium is made of two isotopes 85 Rb and 87 Rb. If 198 grams of rubidium is exposed to air what will be the mass of the product in grams. Alkali metals when exposed to air tarnish quickly due to the.
If 199 grams of rubidium is exposed to air what will be the mass of the product in grams. Rubidium appears as a soft silvery metal. Rubidium is the second most electropositive of the stable alkali elements and liquefies at high ambient temperature 1027 F 393 C.
Rubidium forms four oxides. What are facts about rubidium. The rubidium atom has a radius of 248 pm and a Van der Waals radius of 303 pm.
1799 8718 435 g 999 218. It reacts strongly in water and bursts into flames when exposed to air. When alkali metals are exposed to air get tarnished in air.
Alkali metals react with water to produce heat hydrogen gas and the corresponding metal hydroxide. Correct answer - When rubidium metal is exposed to air two atoms of rubidium Rb combine with one atom of oxygen. A 1798 g.
If 175 grams of rubidium is exposed to air what will be the mass of the product in grams. When exposed to air rubidium spontaneously ignites while with H 2 O it creates an equally volatile reaction. If the air is damp then a hydrolysis may occur liberating hydrogen and forming RbOH and possibly a fire Rb is quite reactive.
Chemistry questions and answers. Like other group 1 elements this metal reacts violently in water. Rubidium forms a number of oxides when exposed to air including rubidium monoxide Rb 2 O Rb 6 O and Rb 9 O 2.
Alkali metals when exposed to air tarnish quickly due to the. In common with potassium and caesium this reaction is usually vigorous enough to ignite the liberated hydrogen. If 216 grams of rubidium is exposed to air what will be the mass of the product in grams.
If 198 grams of rubidium is exposed. Rubidium metal is the second most electropositive of the stable alkali metals. This is due to.
And it is the third most reactive alkali metal. Like the other alkali metals lithium sodium potassium cesium and francium this chemical is highly reactive with both air and water. Shipped in very limited quantities sealed in a copper tube and over packed in a wooden box.
It also undergoes spontaneous ignition when exposed to air. Rubidium-87 is present to the extent of 2785 in natural rubidium and is a beta emitter with a half-life of 49 x 10 10 years.

Rubidium Alchetron The Free Social Encyclopedia

Rubidium Alchetron The Free Social Encyclopedia

Did You Know D I D Did You Know Surface

Rubidium Rb Properties Uses Studiousguy

Rubidium Alchetron The Free Social Encyclopedia

Did You Know There Are Certain Metals Including Potassium Sodium Lithium Rubidium And Caesium That Are So Reactive Tha D I D Did You Know Alkali Metal
Solved When Rubidium Metal Is Exposed To Air Two Atoms Of Chegg Com

Facts About The Elements Rubidium 2019 09 25 Industrial Heating

Ants Are Capable Of Carrying Objects 50 Times Their Own Body Weight Didyouknow Tuesdaythoughts Heavyweight Ant Body Weight Did You Know Body

Rubidium Basic Computer Programming Free Science Lesson Science Chemistry

Rubidium Alchetron The Free Social Encyclopedia

Solved 3 When Rubidium Metal Is Exposed To Air Two Atoms Chegg Com
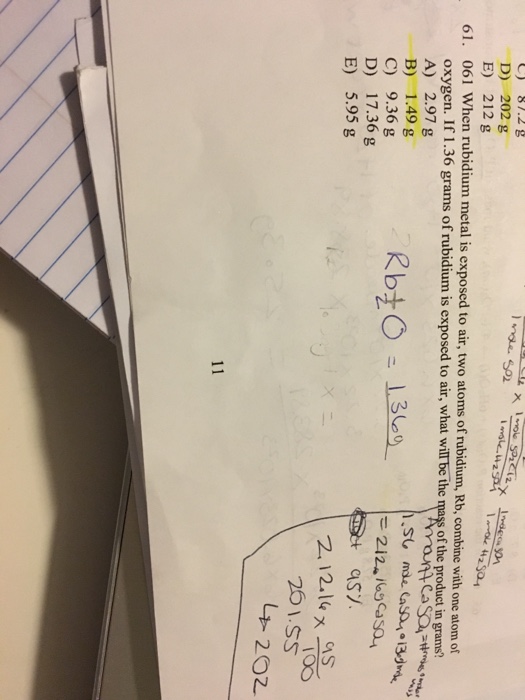
Solved When Rubidium Metal Is Exposed To Air Two Atoms Of Chegg Com

Alkali Metals 19 Reactions Of Rubidium And Caesium With The Air Youtube




Comments
Post a Comment